What Is Represented at the Triple Point of Water
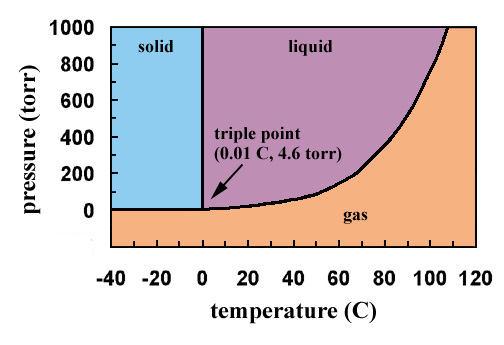
This is the basis of the Kelvin scale and it is equal to 001 о C which is the freezing point of water. For example the water phase diagram has a triple point corresponding to the single temperature and pressure at which solid liquid and gaseous water can coexist in a stable equilibrium 27316 K and a partial vapor pressure of 611657 Pa.
A change in temperature or pressure causes one of the phases to fade away and therefore this measurement is constant.
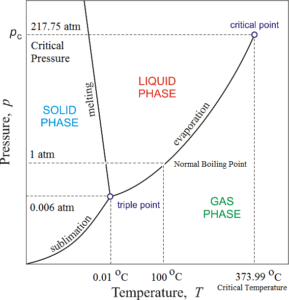
. Figure 772 shows the phase diagram of water and illustrates that the triple point of water occurs at 001C and 000604 atm 459 mmHg. See also Critical Temperatures and Pressures for some common substances. The triple point is merely the point of intersection of the sublimation and vaporization curves.
The triple point of water is a fixed quantity used to define other triple point values and the kelvin unit of temperature. B Three curves OB OA and OC. The most familiar example of a triple point is in water.
In modern thermometry the triple point of water is a standard fixed point. Far more reproducible than the melting point of ice which depends on the amount of dissolved air and the atmospheric pressure the triple point 27316 K is used to define the absolute Kelvin temperature scale. Solid liquid and gas phases in equilibrium B.
Triple points mark conditions at which three different phases can coexist. Solid liquid and supersaturated solution in equilibrium SURMET. The gasliquidsolid triple point of water corresponds to the minimum pressure at which liquid water can exist.
By international agreement the triple point of water has been assigned a value of. More generally the term. In this case the three phases are simply the three usual states of matter.
Above the triple point solid ice when heated at constant pressure first melts to form liquid. For anyone component system the maximum degree of freedom is two. The triple point for water is at 001 degree Celsius at 456 mm Hg.
The triple point temperature is denoted by T 3 and triple point pressure by P 3. Solid ice liquid water and. Its chemical formula is H 2 O.
Simply put the triple point of water is the only temperature at which water can exist in all three states of matter. The triple point is the only condition in which all three phases can coexist. Is it possible for a body to have a negative temperature on the Kelvin scale.
Triple Point of Water Phase diagram of water. The fact that the triple point of a substance is unique is used in modern thermometry. Represented by triple point O.
In other words at the triple point water. The critical point of water is at 647K and 22064 mPa whereas the triple point of water is at 27316 K and 06116557 mPa. The triple point of water is the point at which water can exist at all three states of solid liquid and gas at the same time.
Question 8 of 10 What is represented at the triple point of water. The triple point occurs where the solid liquid and gas transition curves meet. The combination of the temperature and the pressure at which the three phases gas liquid and solid of a substance coexist in thermodynamic equilibrium.
The triple point represents the combination of pressure and temperature that facilitates all phases of matter at equilibrium. Solid gas and vapor molecules changing into each other O. At the pressure and temperature represented by this point all three phases of water coexist in equilibrium.
There are also two important points on the diagram the triple point and the critical point. Water commonly exists in three phases solid liquid and gas. What is represented at the triple point of water.
In the case of ordinary water the triple point is at a pressure of 458 mm Hg and a temperature of 001C. The triple point is merely the point of intersection of the sublimation and vaporization curves It has been found that on a p-T diagram the triple point is represented by a point Fig. Solid ice.
Solid liquid and gas molecules no longer interacting C. The Phase Diagram of Water. On a graph of pressure and temperature the three lines separating the vapourliquid liquidsolid and solidvapour phases all.
At pressures below the triple point as in outer space solid ice when heated at constant pressure is converted directly into water vapor in a process known as sublimation. 193 and on a u-v diagram it is a triangle. Triple point for some common substances.
In thermodynamics the triple point of a substance is the unique combination of temperature and pressure at which solid phase liquid phase and gaseous phase can all coexist in thermodynamic equilibrium. The critical point terminates the liquidgas phase line and relates to the critical pressure the pressure above which a supercritical fluid forms. An example of triple point is the triple point of mercury the triple point of mercury occurs at a temperature of -3883440 degrees Celsius and a pressure of 02 mPa.
Solid liquid and supersaturated solution in equilibrium O B. Solid gas and vapor molecules changing into each other D. So at the triple point of water water exists in three different phases each of which has constant physical properties.
It has been found that on a p-T diagram the triple point is represented by a point and on a p-v diagram it is a line and on a u-v diagram it is a triangle. A Three areas BOC AOC and AOB. C One triple point O.
Each substance including water has a special point called is triple point. The triple point of water is 27316 K at 61173 Pa. This temperature-pressure data pair is called the triple point.
At pressures lower than the triple point water cannot exist as a liquid regardless of the temperature. 196 and on a p-v diagram it is a line Fig. The phase diagram comprises.
Note the triple point may include more than one solid phase if a specific substance has polymorphs. Solid liquid and gas molecules no longer interacting O C.

Change Of Phase Phase Curves And Triple Point Of Water

Critical Point Triple Point Phase Diagrams What Is A Phase Diagram Video Lesson Transcript Study Com

No comments for "What Is Represented at the Triple Point of Water"
Post a Comment